News and Updates

Carolina Liquid Chemistries Now Offers K-ASSAY® CLIA Moderate Complexity Microalbumin Reagent for Use on the Medica EasyRA® Benchtop Clinical Chemistry Analyzer
GREENSBORO, NC — (November 30, 2023) – Carolina Liquid Chemistries Corp. (CLC) has partnered with Kamiya Biomedical Company (Kamiya) to obtain CLIA1 moderate complexity categorization for K-ASSAY® Microalbumin reagent for use on the Medica EasyRA® benchtop clinical chemistry analyzer. CLC distributes the EasyRA in the USA as well as a broad menu of chemistry reagents.
The K-ASSAY Microalbumin reagent is intended for the quantitative determination of human albumin in urine by immunoturbidimetric assay. Measurement of albumin in urine aids in the diagnosis of kidney dysfunction. A small amount of protein is excreted daily into the urine of healthy individuals, making albumin excretion into the urine a useful indicator of early glomerular disease.
Microalbuminuria is a condition characterized by increased urinary excretion of albumin in the absence of overt nephropathy.2,3 Microalbuminuria has been shown to be the earliest stage of diabetic nephropathy in type I diabetes and a marker for development of nephropathy in type II diabetes. Early detection of microalbuminuria may be beneficial in diabetes treatment programs because early detection and management has been shown to reduce the risk and slow progression of end-stage renal disease.4 Albumin in urine has been measured by a variety of methods. The K-ASSAY Microalbumin assay uses an immunoturbidimetric format which provides the necessary sensitivity required for accurate determination of urinary microalbumin.
According to Phillip Shugart, CEO of CLC, “Because of its compact size, ease-of-use, and high speed, the EasyRA is an excellent analyzer for clinical laboratories in small to mid-sized practices, or as a backup analyzer in larger operations.”
CLC offers thirty-five (35) general chemistry assays as well as fourteen (14) screens for drugs of abuse in urine on the EasyRA.
About Carolina Liquid Chemistries
Headquartered in Greensboro, NC, Carolina Liquid Chemistries Corp. (CLC) is an FDA registered manufacturer, re-packager, re-labeler and value-added reseller of chemistry systems and reagents. CLC helps clinical laboratories of all sizes reduce chemistry analyzer and reagent costs, while also receiving accurate and timely results. For more information, visit carolinachemistries.com or email [email protected].
- Clinical Laboratory Improvements Amendments of 1988.
- Morgensen, C.E. and Christensen, C.K., N. Engl. J. Med. 311: 89-93 (1984).
- Viberti, G.C., et al., Lancet. 1430-1432 (1982).
- American Diabetes Association, “Standards of Medical Care in Diabetes-2008”, Diabetes Care 2008 31[Suppl 1]:S29-S30.
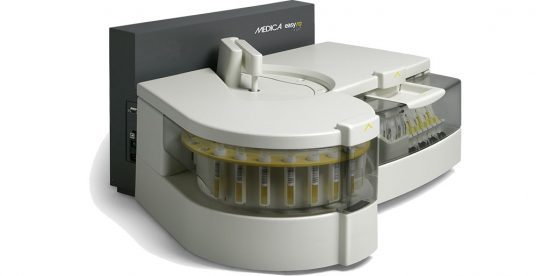
The EasyRA Benchtop Chemistry Analyzer
-
Recent Newsletters
-
Recent Posts
- Carolina Liquid Chemistries Now Offers K-ASSAY® CLIA Moderate Complexity Microalbumin Reagent for Use on the Medica EasyRA® Benchtop Clinical Chemistry Analyzer
- Drug Screening Assays Now Available on the Diazyme DZ-Lite™ c270 Benchtop Clinical Chemistry Analyzer
- The Mission of Our Newsletter
- Carolina Liquid Chemistries Wins Summary Judgment, Defeating False Claim Act Allegation
- Carolina Liquid Chemistries Corp. and Diazyme Laboratories, Inc. Announce Partnership Expanding Test Menu on the Diazyme DZ-Lite™ c270 Benchtop Clinical Chemistry Analyzer, Adding 25 New Assays Totaling Over 45 CLIA Categorized Assays

